Multiple Choice
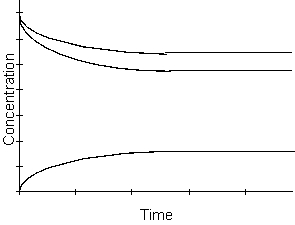
-What happens with respect to time to the products of this reaction in the kinetic region of the graph above?
A) the products increase
B) the products decrease
C) the products remain constant
D) cannot be determined from the graph
Correct Answer:

Verified
Correct Answer:
Verified
Q3: What would be the effect of removing
Q4: Calculate the NO, NO<sub>2</sub>, and O<sub>2</sub> concentrations
Q5: For the following reaction:<br>2 NOCl(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg"
Q6: What is the correct solubility constant expression
Q7: What is the concentration in moles per
Q9: Calculate the concentrations of Cl<sub>2</sub> and ClF<sub>3</sub>
Q10: In which of the following will the
Q11: If the equilibrium constant for the following
Q12: Under which set of conditions must the
Q13: Which is the correct equilibrium constant expression