Multiple Choice
Which of the following equilibria would not be affected by changes in pressure?
A) 2 NO(g) + O2(g) 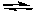 2 NO2(g)
2 NO2(g)
B) N2O4(g) 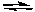 2 NO2(g)
2 NO2(g)
C) 4 NH3(g) + 5 O2(g) 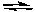 4 NO(g) + 6 H2O(g)
4 NO(g) + 6 H2O(g)
D) CaCO3(s) 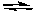 CaO(s) + CO2(g)
CaO(s) + CO2(g)
E) CO(g) + H2O(g) 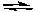 CO2(g) + H2(g)
CO2(g) + H2(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q51: Which one of the following graphs best
Q52: Approximately how many grams of Ag<sub>2</sub>CO<sub>3</sub> will
Q53: Ag<sub>2</sub>SO<sub>4</sub>(s) is in equilibrium with silver and
Q54: If a solution has a Mg<sup>2+</sup> ion
Q55: <br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="
Q57: The following reaction was carried out:<br>Ni(CO)<sub>4</sub>
Q58: Which of the following equations describes
Q59: What is the solubility in moles per
Q60: Describe the relationship between the rate constants
Q61: <br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="