Multiple Choice
Ag2SO4(s) is in equilibrium with silver and sulfate ions in solution:
Ag2SO4(s) 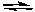 2Ag+(aq) + SO42-(aq)
2Ag+(aq) + SO42-(aq)
Which of the following will not increase the amount of solid Ag2SO4(s) present?
A) boiling off some of the water
B) adding Na2SO4 to the solution
C) adding AgNO3 to the solution
D) adding NaCl to the solution
E) All of the above will increase the amount of Ag2SO4 present.
Correct Answer:

Verified
Correct Answer:
Verified
Q48: What is the K<sub>sp</sub> of PbBr<sub>2</sub> if
Q49: What would happen if O<sub>2</sub> were removed
Q50: Of the compounds in the table
Q51: Which one of the following graphs best
Q52: Approximately how many grams of Ag<sub>2</sub>CO<sub>3</sub> will
Q54: If a solution has a Mg<sup>2+</sup> ion
Q55: <br> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB9692/.jpg" alt="
Q56: Which of the following equilibria would not
Q57: The following reaction was carried out:<br>Ni(CO)<sub>4</sub>
Q58: Which of the following equations describes