Multiple Choice
Refer to the following figure to answer the following questions.
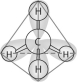
-Which one of the atoms shown would be most likely to form a cation with a charge of +1?
A) 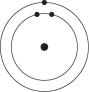
B) 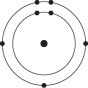
C) 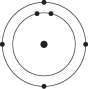
D) 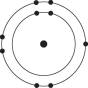
E) 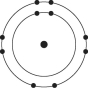
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: The atomic number of sulfur is 16.
Q73: Use the information extracted from the periodic
Q75: Use the information extracted from the periodic
Q76: Draw Lewis structures for each hypothetical
Q77: Refer to the following figure to answer
Q78: How do isotopes of the same element
Q79: Use the information extracted from the periodic
Q80: Use the information extracted from the periodic
Q81: The mass number of an element can
Q82: Use the information extracted from the periodic