Multiple Choice
An ideal monatomic gas expands isothermally from state A to state B.The gas then cools at constant volume to state C.The gas is then compressed isobarically to D before it is heated until it returns to state A.
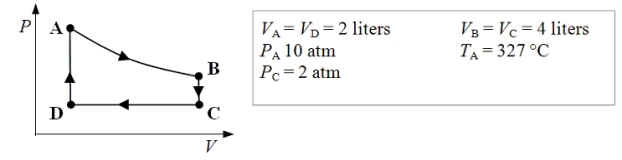
-How much work is done on the gas as it is compressed isobarically from state C to state D?
A) zero joules
B) 50 J
C) 100 J
D) 200 J
E) 400 J
Correct Answer:

Verified
Correct Answer:
Verified
Q9: Complete the following statement: Walls that separate
Q10: An ideal monatomic gas expands isobarically from
Q11: A container is divided into two chambers
Q12: An engine is used to lift a
Q13: An ideal monatomic gas originally in state
Q15: An isobaric process is represented on a
Q16: Neon is a monatomic gas with a
Q17: A fixed amount of ideal gas is
Q18: What is the maximum possible efficiency of
Q19: Which one of the following situations is