Multiple Choice
An ideal monatomic gas originally in state A is taken reversibly to state B along the straight-line path shown in the pressure-volume graph.
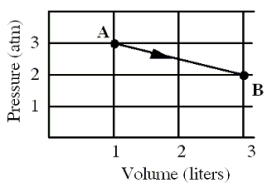
-How much heat,in calories,was exchanged during this process?
A) -110 cal
B) -12 cal
C) zero calories
D) +121 cal
E) +231 cal
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: When the gas enclosed beneath the piston
Q9: Complete the following statement: Walls that separate
Q10: An ideal monatomic gas expands isobarically from
Q11: A container is divided into two chambers
Q12: An engine is used to lift a
Q14: An ideal monatomic gas expands isothermally from
Q15: An isobaric process is represented on a
Q16: Neon is a monatomic gas with a
Q17: A fixed amount of ideal gas is
Q18: What is the maximum possible efficiency of