Multiple Choice
Which is the correct effective solubility constant expression for PbCl2? PbCl2 ⇋ Pb2+ + 2 Cl−
A) 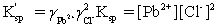
B) 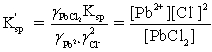
C) 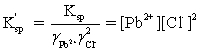
D) 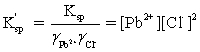
E) 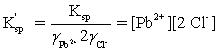
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: The equilibrium constants for a diprotic acid
Q11: A solution is 0.200 M in acetic
Q12: Calculate the pH for a buffer that
Q13: The pH of a solution composed of
Q14: Calculate the concentration of each fumaric acid,HO<sub>2</sub>CCHCHCO<sub>2</sub>H,species
Q15: Express [Ca<sup>2+</sup>] in terms [H<sup>+</sup>] for the
Q16: In addition to the equilibrium CaCO<sub>3</sub> ⇋
Q17: The mass balance equation for the solubility
Q19: The pH of a solution composed of
Q20: Which of the fractional composition equations is