Multiple Choice
Which relationship is NOT correctly defined?
A) Work = E q
B) (G = -n · F · E)
C) 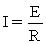
D) P = E I
E) q = n N F
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: For the cell diagram below write out
Q3: Which is true when electrochemical cells are
Q4: E<sup>o</sup><font face="symbol"></font> is defined as:<br>A)the formal half-cell
Q5: A galvanic cell is at equilibrium when:<br>A)E<sub>cell</sub>
Q6: All of the following are true for
Q7: For the line notation below,which is NOT
Q8: For the reaction below,the oxidizing agent is_
Q9: Calculate E<sup>°</sup><font face="symbol"></font> for the half-cell below.
Q10: Calculate the standard potential when the two
Q11: A galvanic cell with a large equilibrium