Multiple Choice
Balance the redox reaction in acid solution: 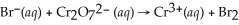 (l)
(l)
A) 4 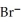 +
+ 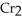
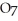 2- + 7
2- + 7 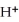 → 2
→ 2 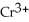 + 2
+ 2 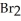 + 7
+ 7 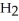 O
O
B) 6 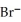 +
+ 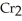
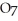 2- + 14
2- + 14 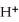 → 2
→ 2 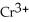 + 3
+ 3 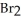 + 7
+ 7 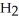 O
O
C) 2 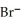 +
+ 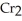
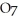 2- + 14
2- + 14 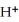 → 2
→ 2 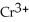 +
+ 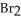 + 7
+ 7 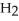 O
O
D) 2 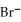 + 2
+ 2 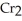
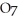 2- + 14
2- + 14 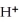 → 4
→ 4 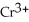 +
+ 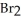 + 14
+ 14 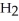 O
O
E) none of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q95: What is the oxidation state of the
Q96: Identify the reducing agent in the following
Q97: Oxidation is the loss of electrons.
Q98: Which substance below would contain a nitrogen
Q99: How many electrons are exchanged when the
Q101: Cars are being developed that run on
Q102: Electrolysis is used to recover many metals
Q103: If you properly balance the following half
Q104: In the following reaction, Mg (s)+ <img
Q105: Reduction can be defined as the gain