True/False
The Lewis structure for the nitrate ion, NO3-, showing all non-zero formal charges is shown here. 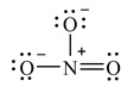
This structure shows a violation of the octet rule.
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q10: Which of the elements listed below would
Q11: Which response includes all the molecules below
Q12: Classify the Ca - Cl bond in
Q13: The Si - Cl bond has less
Q14: The Lewis structure reveals only single bonds
Q16: Which one of the following compounds utilizes
Q17: A Lewis structure for SO<sub>3</sub> that obeys
Q18: Calculate the energy change for the
Q19: Shown here is a Lewis structure for
Q20: A polar covalent bond would form in