Multiple Choice
A Lewis structure for SO3 that obeys the octet rule, showing all non-zero formal charges, is shown here. How many resonance structures for SO3 that obey the octet rule, are possible 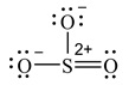
A) only one
B) two
C) three
D) four
E) None of the above
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q12: Classify the Ca - Cl bond in
Q13: The Si - Cl bond has less
Q14: The Lewis structure reveals only single bonds
Q15: The Lewis structure for the nitrate ion,
Q16: Which one of the following compounds utilizes
Q18: Calculate the energy change for the
Q19: Shown here is a Lewis structure for
Q20: A polar covalent bond would form in
Q21: The compound Al(ClO<sub>3</sub>)<sub>3</sub> shows only ionic bonding.
Q22: What is the total number of lone