Multiple Choice
Calculate the value of DS for the reaction shown: 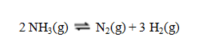 At 25 C the values of entropy in J K-1 mol-1 are ammonia, 192.77; nitrogen, 191.61; and hydrogen, 130.68.
At 25 C the values of entropy in J K-1 mol-1 are ammonia, 192.77; nitrogen, 191.61; and hydrogen, 130.68.
A) +969.19 J/K
B) +393.20 J/K
C) +390.88 J/K
D) +259.03 J/K
E) +198.11 J/K
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q3: According to the Second Law of Thermodynamics,<br>A)
Q4: Which statement is false?<br>A) entropies of gases
Q5: For the reaction shown, DG<sup> <span
Q6: Recovering aluminum directly from its ore, which
Q7: At constant T and P, in which
Q9: If a chemical reaction is at
Q10: Which set of conditions describes a reaction
Q11: Which statement correctly describes the meaning
Q12: Which has the highest entropy at a
Q13: The enthalpy change for a reaction