Essay
Recovering aluminum directly from its ore, which is primarily aluminum oxide, involves the following reaction, for which thermodynamic data is tabulated below: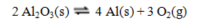

a. Calculate DH°rxn and DS°rxn.
b. Explain how you could have predicted the signs of DH°rxn and DS°rxn without any calculations.
c. Without performing any further calculations, predict whether this reaction will be product-favored only above a certain temperature, or only below a certain temperature. Explain your answer.
d. Calculate the temperature alluded to in Part c.
e. Calculate DG° at this temperature.
Correct Answer:

Verified
a. DH°rxn = (4 ´ 0 + 3 ´ 0) - (2 ´ (-1676.0...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q1: Use the data given to calculate
Q2: Which statement is false?<br>A) entropy is a
Q3: According to the Second Law of Thermodynamics,<br>A)
Q4: Which statement is false?<br>A) entropies of gases
Q5: For the reaction shown, DG<sup> <span
Q7: At constant T and P, in which
Q8: Calculate the value of DS<sup> <span
Q9: If a chemical reaction is at
Q10: Which set of conditions describes a reaction
Q11: Which statement correctly describes the meaning