Multiple Choice
In how many different pairs can these substances be mixed to produce buffer solutions? 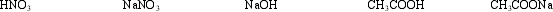
A) 2
B) 3
C) 4
D) 5
E) 6
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q11: Answer the following questions:<br>a. Explain why the solubility
Q12: A 50.00 mL sample of 0.0950 M
Q13: A solution contains 0.100 M Pb(NO<sub>3</sub>)<sub>2</sub> and
Q14: To make a buffer using acetic acid
Q15: What is the largest mass of solid
Q17: The solubility of silver chloride in water
Q18: In which aqueous solution would the smallest
Q19: What volume of 0.1060 M NaOH is
Q20: Exhibit 16-1 The following question(s) refer to
Q21: A weak acid-strong base titration is being