Essay
Answer the following questions:
a. Explain why the solubility of carbonates and phosphates is increased by lowering the pH, but the solubility of chlorides is unaffected by lowering the pH.
b. The solubility of copper(II) carbonate is dramatically increased by the addition of NH3, but the solubility of copper(II) phosphate is not. Referring to the data below, explain why.
Ksp for copper(II) carbonate = 2.5 ´ 10-10
Ksp for copper(II) phosphate = 1.4 ´ 10-37
Cu2+(aq) + 4 NH3(aq)
(Kf = 1.1 ´ 1013)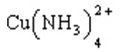
(aq)
Correct Answer:

Verified
a. The carbonate and phosphate ions are ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q6: What is the minimum volume of 0.55
Q7: If solid sodium chloride is added to
Q8: A buffer solution is 0.500 M in
Q9: Isotonic saline solution is 0.154 M NaCl(aq).
Q10: Will a precipitate form when 10.0 mL
Q12: A 50.00 mL sample of 0.0950 M
Q13: A solution contains 0.100 M Pb(NO<sub>3</sub>)<sub>2</sub> and
Q14: To make a buffer using acetic acid
Q15: What is the largest mass of solid
Q16: In how many different pairs can these