Essay
A solution contains 0.100 M Pb(NO3)2 and 1.00 × 10-5 M AgNO3. It is intended to separate out the silver by selective precipitation of AgI. What is the maximum percentage of the total silver that can be recovered free of contamination by PbI2?
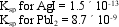
Correct Answer:

Verified
For PbI2, Ksp = [Pb2+][I-]2 Þ [I-] = Ö(Ksp/[Pb2+]). ...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q8: A buffer solution is 0.500 M in
Q9: Isotonic saline solution is 0.154 M NaCl(aq).
Q10: Will a precipitate form when 10.0 mL
Q11: Answer the following questions:<br>a. Explain why the solubility
Q12: A 50.00 mL sample of 0.0950 M
Q14: To make a buffer using acetic acid
Q15: What is the largest mass of solid
Q16: In how many different pairs can these
Q17: The solubility of silver chloride in water
Q18: In which aqueous solution would the smallest