Multiple Choice
The following diagram represents the reaction of A2 (unshaded spheres) with B (shaded spheres) .What is the balanced chemical equation for this reaction,and what is the limiting reactant? 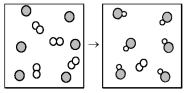
A) A2 + 2B → 2AB;A2 is the limiting reactant.
B) A2 + 2B → 2AB;B is the limiting reactant.
C) 4A2 + 6B → 6AB;A2 is the limiting reactant.
D) 4A2 + 6B → 6AB;B is the limiting reactant.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: How many oxygen atoms are there in
Q17: Which of the following statements about mass
Q74: What is the concentration of NO<sub>3</sub><sup>-</sup> ions
Q75: How many milliliters of a 9.0 M
Q76: 10 g of nitrogen is reacted with
Q77: How many grams of CaCl<sub>2</sub> are formed
Q83: When carbon dioxide dissolves in water,H+ is
Q129: Assume that the unshaded spheres in the
Q147: What is the sum of the coefficients
Q149: What is the molar mass of aspartic