Multiple Choice
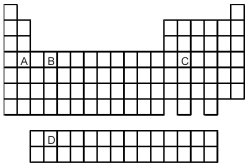
-Which element,indicated by letter on the periodic table above,contains three f electrons?
A) A
B) B
C) C
D) D
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q38: Using shorthand notation,the electron configuration of Ni
Q43: Which of the following elements would you
Q50: How many orbitals are there in the
Q52: What is the ground-state valence-shell electron configuration
Q58: The laser used to read Blu-Ray discs
Q66: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which grouping of
Q99: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -Which grouping of
Q101: An orbital that as the appearance of
Q126: What are the possible values of l
Q155: According to the Balmer-Rydberg equation,the transition from