Multiple Choice
What is the equilibrium equation for the dissociation of formic acid in water?
HCOOH (aq) + H2O (l) ⇌ H3O+ (aq) + HCOO- (aq)
A) Kc = 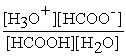
B) Kc = 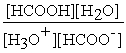
C) Kc = 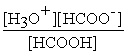
D) Kc = 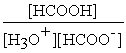
Correct Answer:

Verified
Correct Answer:
Verified
Q15: The following pictures represent the equilibrium state
Q20: The esterification of acetic acid and ethanol
Q84: The reaction below virtually goes to completion
Q109: An equilibrium mixture of CO,O<sub>2</sub> and CO<sub>2</sub>
Q110: The following pictures represent mixtures of cis-C<sub>2</sub>H<sub>2</sub>X<sub>2</sub>
Q111: Phosphorus pentachloride decomposes to phosphorus trichloride at
Q113: Shown below is a concentration vs.time plot
Q119: The equilibrium constant is equal to 5.00
Q122: Oxalic acid can donate two protons to
Q148: The reaction CaCO<sub>3</sub>(s)⇌ CaO(s)+ O<sub>2</sub>(g)is endothermic 298