Multiple Choice
The following pictures represent mixtures of cis-C2H2X2 molecules and trans-C2H2X2 molecules,which interconvert according to the equation cis-C2H2X2 ⇌ trans-C2H2X2.If mixture (1) is at equilibrium,which of the other mixtures are also at equilibrium? 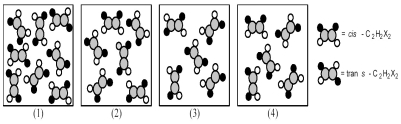
A) mixture (2)
B) mixture (3)
C) mixture (4)
D) None of the other mixtures are at equilibrium.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: For the reaction shown below the equilibrium
Q20: The esterification of acetic acid and ethanol
Q106: Gaseous hydrogen bromide decomposes at elevated temperatures
Q107: Nitric oxide reacts with oxygen to form
Q109: An equilibrium mixture of CO,O<sub>2</sub> and CO<sub>2</sub>
Q111: In a reversible reaction,when the rate of
Q111: Phosphorus pentachloride decomposes to phosphorus trichloride at
Q113: Shown below is a concentration vs.time plot
Q114: What is the equilibrium equation for the
Q148: The reaction CaCO<sub>3</sub>(s)⇌ CaO(s)+ O<sub>2</sub>(g)is endothermic 298