Multiple Choice
The following pictures represent solutions of three salts NaA (A- = X-,Y-,or Z-) ;water molecules and Na+ ions have been omitted for clarity.Arrange the three A- ions in order of decreasing base strength. 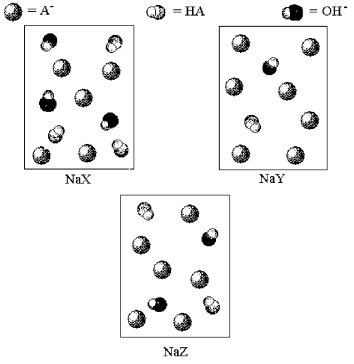
A) X- > Y- > Z-
B) X- > Z- > Y-
C) Y- > Z- > X-
D) Z- > Y- > XZ-
Correct Answer:

Verified
Correct Answer:
Verified
Q6: In the aquation reaction Co<sup>2+</sup> + 6
Q58: The following pictures represent aqueous solutions of
Q76: What is the hydronium ion concentration and
Q79: What is the hydronium ion concentration of
Q80: Which of the following are weak diprotic
Q81: What is the pH of a solution
Q82: Which Br∅nsted-Lowry base has the strongest conjugate
Q83: What is the hydronium ion concentration and
Q129: What is the second stepwise equilibrium constant
Q192: In the following reaction the unshaded spheres