Multiple Choice
For the reaction
2N2O5(g) → 4NO2(g) + O2(g)
Which of the following expressions is equal to the rate of the reaction?
A) 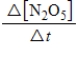
B) 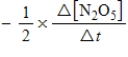
C) 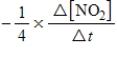
D) 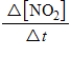
E) 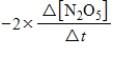
Correct Answer:

Verified
Correct Answer:
Verified
Q65: For the reaction<br>IO<sub>3</sub><sup>-</sup>(aq)+ 5I<sup>-</sup>(aq)+ 6H<sup>+</sup>(aq)→ 3I<sub>2</sub>(aq)+ 3H<sub>2</sub>O(l)<br>The
Q66: Which of the following reactions is not
Q67: In a first-order reaction,the half-life is 139
Q68: The rate constant for a first-order reaction
Q69: For the reaction<br>(CH<sub>3</sub>)<sub>3</sub>CCl(aq)+ OH<sup>-</sup>(aq)→ (CH<sub>3</sub>)<sub>3</sub>COH(aq)+ Cl<sup>-</sup>(aq)<br>It is
Q71: For which order reaction is the half-life
Q72: Which of the following experimental methods cannot
Q73: The OH· radical disproportionates according to the
Q74: Which of the following statements best describes
Q75: The reaction between selenous acid and the