Multiple Choice
The OH· radical disproportionates according to the elementary chemical reaction 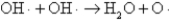 This reaction is second-order in OH·.The rate constant for the reaction is 2.0 × 10-12 cm3/molecules at room temperature.If the initial OH· concentration is 1.4 × 1013 molecules/cm3,what is the first half-life for the reaction?
This reaction is second-order in OH·.The rate constant for the reaction is 2.0 × 10-12 cm3/molecules at room temperature.If the initial OH· concentration is 1.4 × 1013 molecules/cm3,what is the first half-life for the reaction?
A) 2.8 × 101 s
B) 3.6 × 10-2 s
C) 1.8 × 10-2 s
D) 3.5 × 1011 s
E) 7.1 × 10-14 s
Correct Answer:

Verified
Correct Answer:
Verified
Q68: The rate constant for a first-order reaction
Q69: For the reaction<br>(CH<sub>3</sub>)<sub>3</sub>CCl(aq)+ OH<sup>-</sup>(aq)→ (CH<sub>3</sub>)<sub>3</sub>COH(aq)+ Cl<sup>-</sup>(aq)<br>It is
Q70: For the reaction<br>2N<sub>2</sub>O<sub>5</sub>(g)→ 4NO<sub>2</sub>(g)+ O<sub>2</sub>(g)<br>Which of the
Q71: For which order reaction is the half-life
Q72: Which of the following experimental methods cannot
Q74: Which of the following statements best describes
Q75: The reaction between selenous acid and the
Q76: For which of the following hypothetical rate
Q77: Ozone reacts with nitrogen dioxide to produce
Q78: If a reaction is first-order with respect