Multiple Choice
Balance the following half-reaction occurring in acidic solution.
NO3-(aq) → 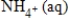
A) NO3-(aq) + 10H+(aq) + 8e− → 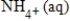 + 3H2O(l)
+ 3H2O(l)
B) NO3-(aq) + 3H2O(l) + 10e− → 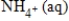 + 10H+(aq)
+ 10H+(aq)
C) NO3-(aq) + 10H+(aq) → 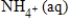 + 3H2O(l) + 10e−
+ 3H2O(l) + 10e−
D) NO3-(aq) + 8e− → 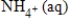 + 8H+(aq) + 3H2O(l)
+ 8H+(aq) + 3H2O(l)
E) NO3-(aq) + 10H+(aq) → 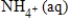 + 3H2O(l)
+ 3H2O(l)
Correct Answer:

Verified
Correct Answer:
Verified
Q2: When the following oxidation-reduction reaction in acidic
Q3: A fuel cell designed to react grain
Q4: Given:<br>Mn<sup>2+</sup>(aq)+ 2e<sup>-</sup> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Given: Mn<sup>2+</sup>(aq)+ 2e<sup>-</sup>
Q5: For a galvanic cell using Fe |
Q6: What is the cell reaction for the
Q7: A voltaic cell is made by placing
Q8: What is one of the major products
Q9: Given:<br>Li<sup>+</sup>(aq)+ e<sup>-</sup> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Given: Li<sup>+</sup>(aq)+ e<sup>-</sup>
Q10: In the following electrochemical cell,what is the
Q11: The process of producing a chemical change