Multiple Choice
Given: 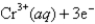

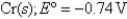
Pb2+ (aq) + 2e-
 Pb(s) ; Eº = -0.13 V
Pb(s) ; Eº = -0.13 V
What is the standard cell potential for the following reaction?
2Cr(s) + 3Pb2+ (aq) → 3Pb(s) + 2Cr3+ (aq)
A) -0.87V
B) 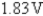
C) -0.61V
D) 0.61V
E) 0.87V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q77: Given: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Given:
Q78: Calculate the solubility product of silver iodide
Q79: What is the logarithm of the equilibrium
Q80: What is the reduction potential for the
Q81: When the following oxidation-reduction reaction in basic
Q83: What is E of the following cell
Q84: In a table of standard reduction potentials,the
Q85: For a reaction in a voltaic cell,both
Q86: Which of the following statements is true
Q87: In order to determine the identity of