Multiple Choice
Given: 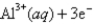

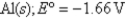
Cl2 (g) + 2e¯  2Cl¯ (aq) ; Eº = 1.36V
2Cl¯ (aq) ; Eº = 1.36V
What is 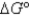
For the following cell reaction?
2AlCl3(aq)  2Al(s) + 3Cl2(g)
2Al(s) + 3Cl2(g)
A) -5.8 × 105 J
B) 5.8 × 105 J
C) 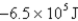
D) -1.7 × 106 J
E) 1.7 × 106 J
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q72: What reaction occurs at the anode during
Q73: Cathodic protection results when<br>A)iron is amalgamated with
Q74: How many moles of electrons are produced
Q75: What is the correct cell notation for
Q76: A voltaic cell is made by placing
Q78: Calculate the solubility product of silver iodide
Q79: What is the logarithm of the equilibrium
Q80: What is the reduction potential for the
Q81: When the following oxidation-reduction reaction in basic
Q82: Given: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB2288/.jpg" alt="Given: