Multiple Choice
The combustion of titanium with oxygen produces titanium dioxide:
Ti(s) + O2(g) →TiO2 (s)
When 2.060 g of titanium is combusted in a bomb calorimeter,the temperature of the calorimeter increases from 25.00 °C to 91.60 °C.In a separate experiment,the heat capacity of the calorimeter is measured to be 9.84 kJK-1 .The heat of reaction for the combustion of a mole of Ti in this calorimeter is ________ kJ mol-1.
A) 14.3
B) 19.6
C) -311
D) -0.154
E) -1.52 × 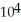
Correct Answer:

Verified
Correct Answer:
Verified
Q11: Calculate the standard enthalpy of formation of
Q12: Heat is usually transferred from a cold
Q13: When 2.50 g of Ba(s)is added to
Q14: How much heat is absorbed/released when 40.00
Q15: What is the final temperature in the
Q17: What is the work done in joules
Q18: What is the reaction for the standard
Q19: Calculate the amount of heat absorbed by
Q20: When power was turned off to a
Q21: The specific heat capacity of methane gas