Multiple Choice
How much heat is absorbed/released when 40.00 g of NH3(g) reacts in the presence of excess O2(g) to produce NO(g) and H2O(l) according to the following chemical equation?
4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(l) 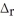 H° = 1168 kJ
H° = 1168 kJ
A) 685.8 kJ of heat are absorbed.
B) 685.8 kJ of heat are released.
C) 2743 kJ of heat are absorbed.
D) 2743 kJ of heat are released.
Correct Answer:

Verified
Correct Answer:
Verified
Q9: The final temperature when 150 mL of
Q10: Lead,water,sulfur and arsenic have specific heats of
Q11: Calculate the standard enthalpy of formation of
Q12: Heat is usually transferred from a cold
Q13: When 2.50 g of Ba(s)is added to
Q15: What is the final temperature in the
Q16: The combustion of titanium with oxygen produces
Q17: What is the work done in joules
Q18: What is the reaction for the standard
Q19: Calculate the amount of heat absorbed by