Multiple Choice
When 2.50 g of Ba(s) is added to 100.00 g of water in a container open to the atmosphere,the reaction shown below occurs and the temperature of the resulting solution rises from 22.00 °C to 40.32 °C.If the specific heat of the solution is 4.18 J g-1°C-1 ,calculate 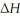 For the reaction,as written.
For the reaction,as written.
Ba(s) + 2 H2O(l) → Ba(OH) 2(aq) + H2(g)
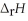 = ?
= ?
A) -431 kJ mol-1
B) -3.14 kJ mol-1
C) +3.14 kJ mol-1
D) +431 kJ mol-1
Correct Answer:

Verified
Correct Answer:
Verified
Q8: The complete combustion of 1 mole of
Q9: The final temperature when 150 mL of
Q10: Lead,water,sulfur and arsenic have specific heats of
Q11: Calculate the standard enthalpy of formation of
Q12: Heat is usually transferred from a cold
Q14: How much heat is absorbed/released when 40.00
Q15: What is the final temperature in the
Q16: The combustion of titanium with oxygen produces
Q17: What is the work done in joules
Q18: What is the reaction for the standard