Multiple Choice
Consider the reaction: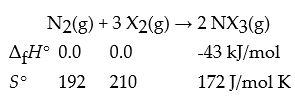
What is ΔrG° for this reaction at 591 K? Is the reaction spontaneous at 591 K?
A) -196 kJ/mol,no
B) 196 kJ/mol,yes
C) 196 kJ/mol,no
D) 239 kJ/mol,yes
E) -239 kJ/mol,no
Correct Answer:

Verified
Correct Answer:
Verified
Q58: The change in Gibbs energy of a
Q59: Predict whether ΔS is positive or negative
Q60: Non spontaneous reactions and spontaneous reactions cannot
Q61: What is ?<sub>r</sub>G°?<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="What is ?<sub>r</sub>G°?
Q62: For a reaction K<sub>eq</sub> = 1.2 ×
Q64: For the reaction,N<sub>2</sub>O<sub>4</sub>(g)→ 2 NO<sub>2</sub>(g)<br>S° (J/mol K)304.2
Q65: Consider the reaction:<br>N<sub>2</sub>(g)+ 3 X<sub>2</sub>(g)→ 2 NX<sub>3</sub>(g)<br>△<sub>f</sub>H°
Q66: Calculate the total quantity of heat required
Q67: Consider the following reaction.<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="Consider the
Q68: Consider the reaction of 25.0 mL of