Multiple Choice
Calculate the total quantity of heat required to convert 25.0 g of liquid CCl4(l) at 35.0 °C to gaseous CCl4 at 76.8 °C (the normal boiling point for CCl4) .The specific heat of CCl4(l) is 0.857 Jg-1°C-1 Its heat of fusion is 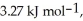 And its heat of vaporization is
And its heat of vaporization is 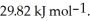
A) 0.896 kJ
B) 1.43 kJ
C) 5.74 kJ
D) 6.28 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Q61: What is ?<sub>r</sub>G°?<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="What is ?<sub>r</sub>G°?
Q62: For a reaction K<sub>eq</sub> = 1.2 ×
Q63: Consider the reaction:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="Consider the reaction:
Q64: For the reaction,N<sub>2</sub>O<sub>4</sub>(g)→ 2 NO<sub>2</sub>(g)<br>S° (J/mol K)304.2
Q65: Consider the reaction:<br>N<sub>2</sub>(g)+ 3 X<sub>2</sub>(g)→ 2 NX<sub>3</sub>(g)<br>△<sub>f</sub>H°
Q67: Consider the following reaction.<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="Consider the
Q68: Consider the reaction of 25.0 mL of
Q69: Which of the following quantities is practically
Q70: Which of the following statements are true?<br>I.Liquids
Q71: Phosphorus and chlorine gases combine to produce