Multiple Choice
Consider the following reaction.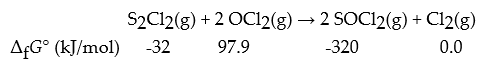
What is ΔrG° for this reaction in kJ?
A) -804
B) -476
C) -386
D) -413
E) -799
Correct Answer:

Verified
Correct Answer:
Verified
Q62: For a reaction K<sub>eq</sub> = 1.2 ×
Q63: Consider the reaction:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="Consider the reaction:
Q64: For the reaction,N<sub>2</sub>O<sub>4</sub>(g)→ 2 NO<sub>2</sub>(g)<br>S° (J/mol K)304.2
Q65: Consider the reaction:<br>N<sub>2</sub>(g)+ 3 X<sub>2</sub>(g)→ 2 NX<sub>3</sub>(g)<br>△<sub>f</sub>H°
Q66: Calculate the total quantity of heat required
Q68: Consider the reaction of 25.0 mL of
Q69: Which of the following quantities is practically
Q70: Which of the following statements are true?<br>I.Liquids
Q71: Phosphorus and chlorine gases combine to produce
Q72: If the vapor pressure of water in