Multiple Choice
Phosphorus and chlorine gases combine to produce phosphorus trichloride: 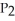 (g) +
(g) + 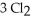 (g) →
(g) → 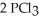 (g)
(g)
ΔrG° at 298 K for this reaction is -642.9 kJ mol-1.The value of ΔrG at 298 K for a reaction mixture that consists of 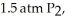
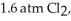 And
And 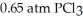 Is ________ kJ mol-1.
Is ________ kJ mol-1.
A) -44.2
B) -3.88 × 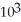
C) -7.28 × 103
D) -708.4
E) -649.5
Correct Answer:

Verified
Correct Answer:
Verified
Q66: Calculate the total quantity of heat required
Q67: Consider the following reaction.<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5343/.jpg" alt="Consider the
Q68: Consider the reaction of 25.0 mL of
Q69: Which of the following quantities is practically
Q70: Which of the following statements are true?<br>I.Liquids
Q72: If the vapor pressure of water in
Q73: Ethyl chloride,C<sub>2</sub>H<sub>5</sub>Cl,is used as a local anesthetic.It
Q74: The value of Δ<sub>f</sub>G at 100.0 °C
Q75: In a reversible reaction,a forward spontaneous reaction
Q76: Which one of the following would be