Multiple Choice
In the equilibrium system described by: 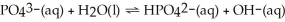
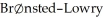 Theory would designate:
Theory would designate:
A) PO43- and H2O as the bases
B) H2O and OH- as a conjugate pair
C) HPO42- and OH- as the acids
D) HPO42- and H2O as a conjugate pair
E) PO43- as amphiprotic
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q27: List the following acids in order of
Q28: What is the pH of a 0.380
Q29: Calculate the pH of a 1.60 mol
Q30: What is the pH of a 0.040
Q31: What is the [H<sub>2</sub>AsO<sub>4</sub><sup>-</sup>] for an aqueous
Q33: In the reaction BF<sub>3</sub> + NH<sub>3</sub> →
Q34: What is the pH of a 0.361
Q35: List the following acids in order of
Q36: According to the Arrhenius theory,a neutralization reaction
Q37: What is the pH of a 0.500