Multiple Choice
A gas is allowed to expand, at constant temperature, from a volume of 1.0 L to 10.1 L against an external pressure of 0.50 atm.If the gas absorbs 250 J of heat from the surroundings, what are the values of q, w, and E? 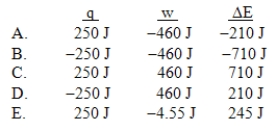
A) A
B) B
C) C
D) D
E) E
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q60: The heat of solution of LiCl is
Q98: The heat of solution of KCl is
Q99: At 25°C, the following heats of
Q101: Given the following <span class="ql-formula"
Q102: Calcium oxide and water react in
Q103: Calculate the standard enthalpy change for
Q105: Butane (C<sub>4</sub>H<sub>10</sub>)undergoes combustion in excess oxygen
Q106: A 100.mL sample of 0.200 M
Q107: At 25°C, the standard enthalpy of
Q109: Calculate the amount of work done, in