Short Answer
Given the following H° values,
H2(g)+ 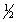 O2(g) H2O(l), H°f = -285.8 kJ/mol
O2(g) H2O(l), H°f = -285.8 kJ/mol
H2O2(l), H2(g)+ O2(g), H°rxn = 187.6 kJ/mol
calculate H°rxn for the reaction H2O2(l) H2O(l)+ 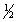 O2(g),
O2(g),
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q60: The heat of solution of LiCl is
Q96: Which of the following has a
Q97: Suppose a 50.0 g block of silver
Q98: The combustion of pentane produces heat
Q99: At 25°C, the following heats of
Q102: Calcium oxide and water react in
Q103: Calculate the standard enthalpy change for
Q104: A gas is allowed to expand,
Q105: Butane (C<sub>4</sub>H<sub>10</sub>)undergoes combustion in excess oxygen
Q106: A 100.mL sample of 0.200 M