Multiple Choice
At 25°C, the following heats of reaction are known: 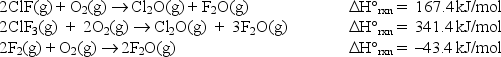
At the same temperature, use the above data to calculate the heat released (kJ) when 3.40 moles of ClF(g) reacts with excess F2: ClF(g) + F2(g) ClF3(g)
A) 109 kJ
B) 233 kJ
C) 370.kJ
D) 465 kJ
E) 1,580 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q60: The heat of solution of LiCl is
Q94: Calculate the heat required when 2.50
Q95: When 18.5 g of HgO(s)is decomposed
Q96: Which of the following has a
Q97: Suppose a 50.0 g block of silver
Q98: The combustion of pentane produces heat
Q101: Given the following <span class="ql-formula"
Q102: Calcium oxide and water react in
Q103: Calculate the standard enthalpy change for
Q104: A gas is allowed to expand,