Multiple Choice
Sulfur can be separated from lead in the mineral galena, PbS(s) , by "roasting" the ore in the presence of oxygen as shown in the following reaction: 2PbS(s) + 3O2(g) 2PbO(s) + 2SO2(g)
Calculate S° for this reaction using the thermodynamic data provided below. 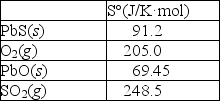
A) -410 J/K·mol
B) -161.5 J/K·mol
C) -47.7 J/K·mol
D) 21.8 J/K·mol
E) 43.5 J/K·mol
Correct Answer:

Verified
Correct Answer:
Verified
Q53: Using the thermodynamic data provided below, calculate
Q54: Predict the sign of <span
Q56: For the reaction 3H<sub>2</sub>(g)+ N<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q57: For the reaction CuS(s)+ H<sub>2</sub>(g) <img
Q59: Arrange the following substances in the
Q60: How does the entropy change when a
Q61: Which species will have the lowest absolute
Q63: Using the thermodynamic data provided below, calculate
Q96: Sodium carbonate can be made by
Q122: Which response includes all of the