Essay
Using the thermodynamic data provided below, calculate the standard change in entropy when one mole of sodium sulfate is dissolved in water? 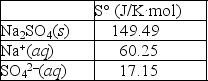 Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Correct Answer:

Verified
-11.84 J/K·mol; solu...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q7: Which of these species would you expect
Q48: Consider the reaction CO(g)+ 2H<sub>2</sub>(g) <img
Q50: Determine <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q54: Predict the sign of <span
Q56: For the reaction 3H<sub>2</sub>(g)+ N<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q57: For the reaction CuS(s)+ H<sub>2</sub>(g) <img
Q58: Sulfur can be separated from lead
Q64: Melting an ionic solid always results in
Q77: Which of these species would you expect
Q96: Sodium carbonate can be made by