Short Answer
For the reaction 3H2(g)+ N2(g) 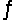 2NH3(g), Kc = 9.0 at 350°C. In what direction does the reaction proceed when 1.0 mol NH3, 5.0 mol N2, and 5.0 mol H2 are mixed in a 2.5 L reactor?
2NH3(g), Kc = 9.0 at 350°C. In what direction does the reaction proceed when 1.0 mol NH3, 5.0 mol N2, and 5.0 mol H2 are mixed in a 2.5 L reactor?
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q53: Using the thermodynamic data provided below, calculate
Q54: Predict the sign of <span
Q57: For the reaction CuS(s)+ H<sub>2</sub>(g) <img
Q58: Sulfur can be separated from lead
Q60: How does the entropy change when a
Q61: Which species will have the lowest absolute
Q64: Melting an ionic solid always results in
Q77: Which of these species would you expect
Q96: Sodium carbonate can be made by
Q122: Which response includes all of the