Short Answer
Consider the reaction CO(g)+ 2H2(g) 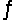 CH3OH(l)at 25°C.
CH3OH(l)at 25°C.
G°f (CO)= -137.3 kJ/mol
G°f (CH3OH)= -166.3 kJ/mol
H°f (CO)= -110.5 kJ/mol
H°f (CH3OH)= -238.7 kJ/mol
S°(CO)= 197.9 J/K·mol
S°(CH3OH)= 126.8 J/K·mol
Calculate G° at 25°C.
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Which of these species has the highest
Q15: Is the reaction SiO<sub>2</sub>(s)+ Pb(s) <span
Q102: Calculate <span class="ql-formula" data-value="\Delta"><span class="katex"><span
Q103: The equilibrium constant for the reaction
Q104: The equilibrium constant at 427°C for
Q105: Find the temperature at which K<sub>p</sub>
Q109: Using the thermodynamic data provided below, calculate
Q111: For the reaction 3H<sub>2</sub>(g)+ N<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3245/.jpg"
Q112: Given the following data, estimate the
Q128: Which one of the following reactions