True/False
The following two structural formulas represent isomers. 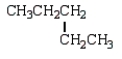
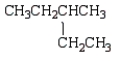
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Which of the following elements has the
Q3: The maximum number of electrons that a
Q9: How many electrons are there in the
Q56: Which of the following best represents the
Q57: Circle all of the sp hybridized atoms
Q60: Draw bond-line structures of all of
Q61: Consider the following structural formula. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1813/.jpg"
Q63: Which of the following is trigonal planar?<br>A)boron
Q87: What is the ground-state electronic configuration of
Q98: The percent s character in an sp<sup>2</sup>