Essay
Circle all of the sp hybridized atoms in the following molecular structure. 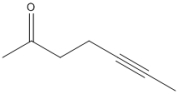
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Which of the following elements has the
Q3: The maximum number of electrons that a
Q52: Provide a neatly drawn figure to show
Q52: Rank the following in order of decreasing
Q56: Which of the following best represents the
Q59: The following two structural formulas represent isomers.
Q60: Draw bond-line structures of all of
Q61: Consider the following structural formula. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB1813/.jpg"
Q87: What is the ground-state electronic configuration of
Q98: The percent s character in an sp<sup>2</sup>