True/False
Consider the following structural formula. 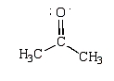 The following is a resonance structure.
The following is a resonance structure. 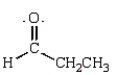
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: Which of the following elements has the
Q9: How many electrons are there in the
Q56: Which of the following best represents the
Q57: Circle all of the sp hybridized atoms
Q59: The following two structural formulas represent isomers.
Q60: Draw bond-line structures of all of
Q63: Which of the following is trigonal planar?<br>A)boron
Q65: Circle and name the functional groups in
Q75: Which of the following molecules has a
Q98: The percent s character in an sp<sup>2</sup>