Multiple Choice
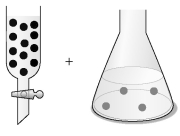
-The concentration of an aqueous solution of I3⁻ can be determined by a redox titration with aqueous sodium thiosulfate,Na2S2O3: 2 S2O32- (aq) + I3⁻ (aq) + → S4O62- (aq) + 3 I⁻ (aq)
Assume that the black spheres in the buret represent S2O32- ions,the gray spheres in the flask represent I3- ions,the concentration of the S2O32- ions in the buret is 0.120 M,and the volumes in the buret and the flask are identical.What is the concentration of the I3- in the flask,and what fraction of the S2O32- solution in the buret must be added to the flask to react with all the I3- ions?
A) 0.0400 M I3-;1/3 of the S2O32- must be added.
B) 0.0400 M I3-;2/3 of the S2O32- must be added.
C) 0.0600 M I3-;1/3 of the S2O32- must be added.
D) 0.0600 M I3-;2/3 of the S2O32- must be added.
Correct Answer:

Verified
Correct Answer:
Verified
Q144: When 200.mL of 1.50 × 10<sup>-4</sup> M
Q145: A student prepared a stock solution by
Q146: Which of the following is not a
Q147: Predict the products of a reaction between
Q148: When 50.0 mL of a 1.00 M
Q150: Assume that the conductivity of a solution
Q151: The compound K<sub>2</sub>CO<sub>3</sub> is predicted to be
Q152: Write a net ionic equation for the
Q153: Which one of the following compounds is
Q154: Ascorbic acid,C<sub>6</sub>H<sub>8</sub>0<sub>6</sub>,can be represented by the molecular