Multiple Choice
Ascorbic acid,C6H806,can be represented by the molecular model shown below.If 1.00 mol of ascorbic acid is submitted to combustion analysis,how many moles of CO2 and how many moles of H2O would be formed? 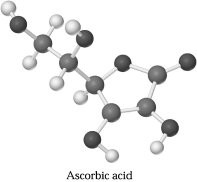
A) 3.00 mol CO2 and 2.00 mol H2O
B) 6.00 mol CO2 and 4.00 mol H2O
C) 6.00 mol CO2 and 8.00 mol H2O
D) 12.0 mol CO2 and 10.0 mol H2O
Correct Answer:

Verified
Correct Answer:
Verified
Q149: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4940/.jpg" alt=" -The concentration of
Q150: Assume that the conductivity of a solution
Q151: The compound K<sub>2</sub>CO<sub>3</sub> is predicted to be
Q152: Write a net ionic equation for the
Q153: Which one of the following compounds is
Q155: Because it forms some H<sup>+</sup> and OCl<sup>-</sup>
Q156: Which contains the greatest number of bromide
Q157: When dissolved in water,of HClO<sub>4</sub>,NH<sub>3</sub>,KOH,HI,and CH<sub>3</sub>OH which
Q158: The best oxidizing agents are found at
Q159: If 200.mL of 0.100 M Na<sub>2</sub>SO<sub>4</sub> is