Multiple Choice
The following MO diagram is appropriate for Li2 and Be2.Based on this diagram, 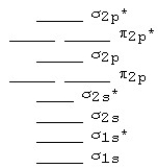
A) both are stable and diamagnetic.
B) Li2 is stable and diamagnetic,but Be2 is unstable.
C) Be2 is stable and diamagnetic,but Li2 is unstable.
D) Be2 is stable and paramagnetic,but Li2 is unstable.
Correct Answer:

Verified
Correct Answer:
Verified
Q131: The polarity of CCl<sub>4</sub> is _.
Q132: What is the geometry around the central
Q133: Helium can be liquefied when He atoms
Q134: What is the geometry around the central
Q135: What are the bond angles in the
Q137: Identify the set of hybrid orbitals shown
Q138: Shown below is a model of CBr<sub>4</sub>
Q139: Which of the following would be expected
Q140: Which compound below could have a zero
Q141: What is the molecular geometry of TeBr<sub>4</sub>?<br>A)seesaw<br>B)square