Multiple Choice
What are the bond angles in the following molecular model of XeF4? 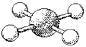
A) some less than 90° and some less than 120° but greater than 90°
B) 90°
C) some less than 90° and some less than 180° but greater than 90°
D) 90° and 180°
Correct Answer:

Verified
Correct Answer:
Verified
Q130: What is the molecular geometry of SeF<sub>5</sub><sup>-</sup>?<br>A)octahedral<br>B)seesaw<br>C)square
Q131: The polarity of CCl<sub>4</sub> is _.
Q132: What is the geometry around the central
Q133: Helium can be liquefied when He atoms
Q134: What is the geometry around the central
Q136: The following MO diagram is appropriate for
Q137: Identify the set of hybrid orbitals shown
Q138: Shown below is a model of CBr<sub>4</sub>
Q139: Which of the following would be expected
Q140: Which compound below could have a zero