Multiple Choice
What is the geometry around the central atom in the following molecular model of NO2-? 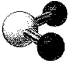
A) linear
B) bent
C) trigonal planar
D) trigonal pyramidal
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q129: Which has the smallest dipole-dipole forces?<br>A)CH<sub>3</sub>F<sub> </sub><br>B)HCl<sub>
Q130: What is the molecular geometry of SeF<sub>5</sub><sup>-</sup>?<br>A)octahedral<br>B)seesaw<br>C)square
Q131: The polarity of CCl<sub>4</sub> is _.
Q132: What is the geometry around the central
Q133: Helium can be liquefied when He atoms
Q135: What are the bond angles in the
Q136: The following MO diagram is appropriate for
Q137: Identify the set of hybrid orbitals shown
Q138: Shown below is a model of CBr<sub>4</sub>
Q139: Which of the following would be expected