Multiple Choice
Which ion-dipole interaction results in the larger (more negative) hydration energy? 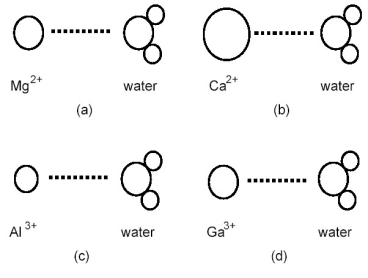
A) diagram (a)
B) diagram (b)
C) diagram (c)
D) diagram (d)
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q57: Arrows in the energy diagram below represent
Q64: The number of moles of ions in
Q65: At 80.0°C benzene has a vapor pressure
Q66: What is the weight percent of a
Q67: In most liquid solutions,the component present in
Q68: For the process of dissolving a solid
Q70: A KCl solution is prepared by dissolving
Q71: Aqueous solutions of 30% (by weight)hydrogen peroxide,H<sub>2</sub>O<sub>2</sub>,are
Q72: Drawing (1)shows a system in which an
Q73: Calculate the freezing point of a solution